43 label chemical equation
Chemical Equations Flashcards | Quizlet Chemical Equation. symbolic representation of a chemical reaction, will show the same # of each type of atom on each side of the equation. ex. Na + Cl2 -> NaCl. Chemical Formula. symbolic representation of of an element or compound. ex. NaCl (table salt) Chemical Reaction. process in which bonds between atoms are broken and new bonds are formed. Label Chemical Equations - Labelled diagram Label Chemical Equations. Share Share by Garyandmarci. G8 Science. Show More. Like. Edit Content. Embed. More. Leaderboard. Show more Show less . This leaderboard is currently private. Click Share to make it public. This leaderboard has been disabled by the resource owner. This leaderboard is disabled as your options are different to the ...
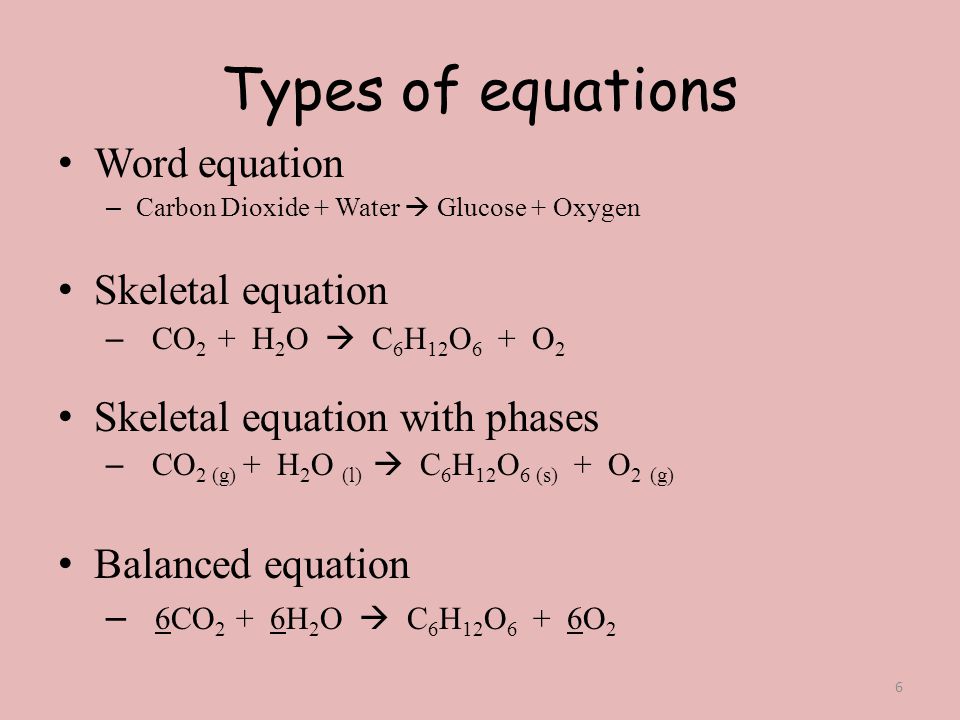
3.1: Chemical Equations - Chemistry LibreTexts The balanced chemical equation for the combustion of glucose in the laboratory (or in the brain) is as follows: C 6H 12O 6(s) + 6O 2(g) → 6CO 2(g) + 6H 2O(l) Construct a table showing how to interpret the information in this equation in terms of. a single molecule of glucose. moles of reactants and products.
Label chemical equation
Al + O2 = Al2O3 - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a Al + b O 2 = c Al 2 O 3. Create a System of Equations. Create an equation for each element (Al, O) where each term represents the number of atoms of the element in each reactant or product. Al: 1 a + 0b = 2 c O: 0a + 2 b = 3 c ... How to Write a Chemical Equation (with Pictures) - wikiHow If you want to write a chemical equation, start by writing the chemical formulas of each reactant. Use the prefixes, such as mono-, di-, tri-, and tetra-, to figure out the number of atoms present for each element, and write this number as a subscript for each element. For example, dihydrogen monoxide would be more easily written as H2O. N2 + H2 = NH3 - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a N 2 + b H 2 = c NH 3. Create a System of Equations. Create an equation for each element (N, H) where each term represents the number of atoms of the element in each reactant or product. N: 2 a + 0b = 1 c H: 0a + 2 b = 3 c; Solve ...
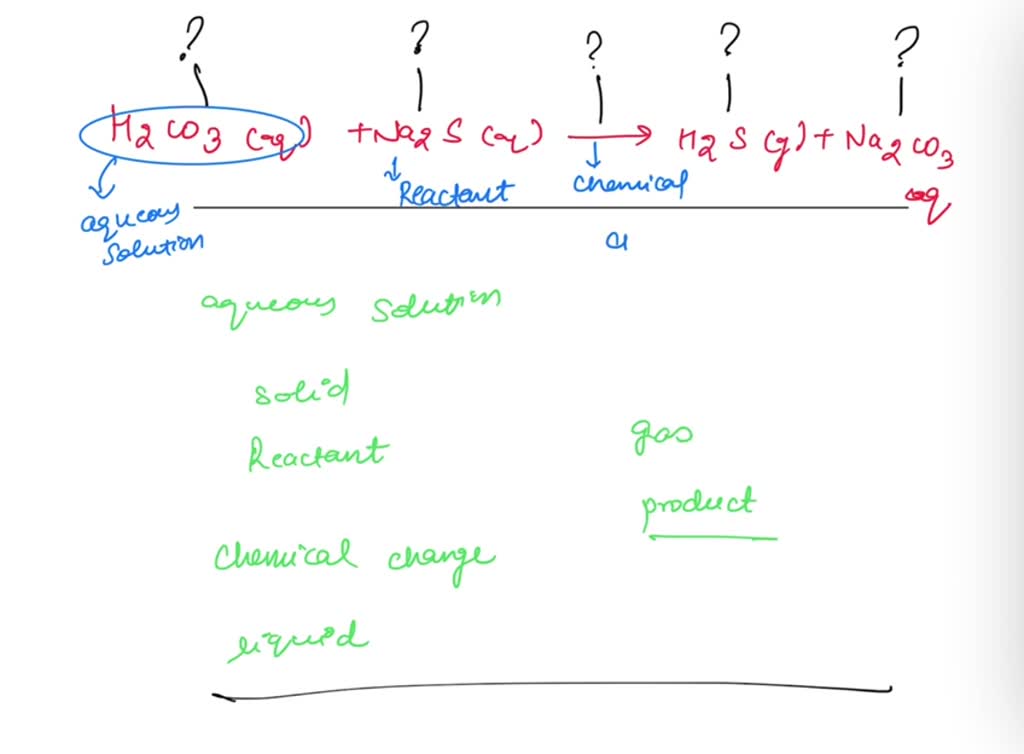
Label chemical equation. Solved label the parts of the chemical equation with the | Chegg.com Expert Answer. Transcribed image text: On 7 of 29 Label the parts of the chemical equation with the appropriate descriptions. MnCl, (aq) + 2 KOH (aq) Mn (OH), (s) + 2 KCl (aq) Answer Bank aqueous solution product solid reactant liquid chemical change. Cellular Respiration Equation, Types, Stages, Products & Diagrams Cellular Respiration Equation: C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + 38*ATP. 10. Cellular Respiration Equation: Every machine needs specific parts and fuel to function. Likewise, " biological machines " also require well-engineered parts and a good energy source to work. Perhaps the second most important molecule (DNA is the first) is ... Chemical Equation | Reactants And Products In Chemical Reactions - BYJUS The representation of a chemical reaction in the form of symbols (substances) is known as chemical equation. A chemical equation consists of reactants, products and an arrow showing the direction of reaction. The equation in which number of atoms of all the molecules is equal on both sides of the equation is known as balanced chemical equation ... P2O5 + H2O = H3PO4 - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a P 2 O 5 + b H 2 O = c H 3 PO 4. Create a System of Equations. Create an equation for each element (P, O, H) where each term represents the number of atoms of the element in each reactant or product. P: 2 a + 0b = 1 c O: 5 a + 1 b ...
3.10: Writing and Balancing Chemical Equations In a balanced chemical equation, both the numbers of each type of atom and the total charge are the same on both sides. Equations \ (\ref {3.1.1}\) and \ (\ref {3.1.2}\) are balanced chemical equations. What is different on each side of the equation is how the atoms are arranged to make molecules or ions. WebMD Drugs & Medications - Medical information on … WebAnswer your medical questions on prescription drugs, vitamins and Over the Counter medications. Find medical information, terminology and advice including side effects, drug interactions, user ... What are the Parts of a Chemical Equation? | Life Persona The steps followed to write a chemical equation are: - Reagents and reaction products are identified and noted. - The formula or symbols of the reagents are written on the left side with a '+' sign between them. - The formula (s) of the products are written on the right side with a '+' sign between them. How do you label chemical equations? [FAQs!] The label on an original chemical container must be legible and written in English. It must include the chemical/product name as shown on the SDS and the. ... How do you label chemical equations? October 10, 2022 September 7, 2022 by Alexander. READ SOMETHING ELSE. Table of Contents show
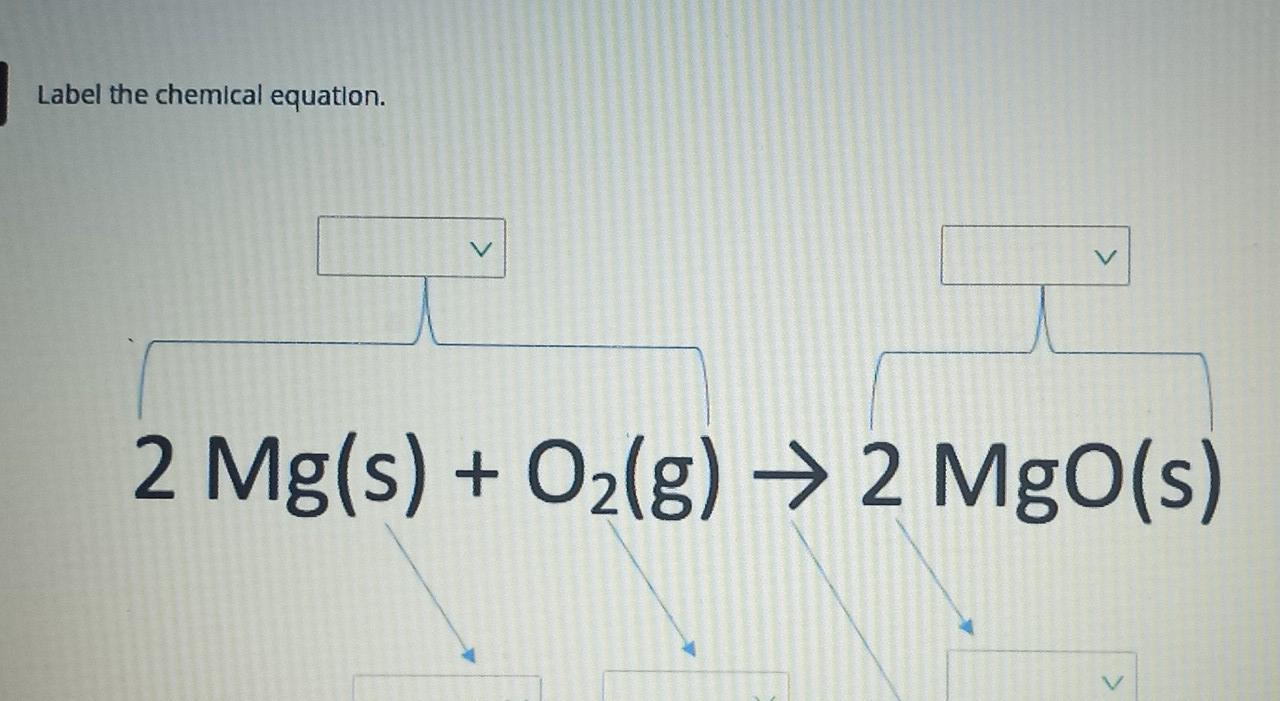
Higher Education Support | McGraw Hill Higher Education WebLearn more about McGraw-Hill products and services, get support, request permissions, and more. Chemical Equation Balancer To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Examples: Fe, Au, Co, Br, C, O, N, F. Ionic charges are not yet supported and will be ignored. How to Number or Label Equations in Microsoft Word - How-To Geek Open your document and select your first equation. On the References tab, click "Insert Caption" from the Captions section of the ribbon. In the Caption pop-up window, select "Equation" next to Label. This sets both the word and the number as the caption. Optionally, select a Position for the caption and click "OK" to apply the caption. Label the chemical equation. 2 Mg(s) - Brainly.com Given the chemical equation: 2 Mg(s) + O2(g) → 2 MgO(s) 2 molecules of Magnesium + Oxygen gas (Reactant) → ( yield) 2 molecules of magnesium oxide (product) 2 in Mg and MgO (coefficients) 2 in Oxygen gas (subscript) Solid (s) - physical state of Reactant Mg. Gas (g) - physical state of Reactant O2. Solid (s) - physical state of product 2MgO
What is a Chemical Equation? - Definition & Examples A chemical equation provides information about the correct proportions of ingredients that are needed to make new substances in chemical reactions. To make a large glass of lemonade, use 1 1/2 ...
What are Chemical Equations? Detailed Explanation, Examples - BYJUS An example of an ionic chemical equation is provided below. Chemical Equation: CaCl 2 + 2AgNO 3 → Ca (NO 3) 2 + 2AgCl↓. Ionic Equation: Ca 2+ + 2Cl - + 2Ag + + 2NO 3- → Ca 2+ + 2NO 3- + 2AgCl↓. Comparing the reactants and the products of the ionic equation and the chemical equation, it can be observed that the Ca 2+ ( calcium ion ...
Corteva Agriscience™ | United States WebEnriching the lives of those producing and consuming our global food supply. From seeds and crop protection to software and services, we're committed to agriculture.
HCl + NaOH = NaCl + H2O - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a HCl + b NaOH = c NaCl + d H 2 O. Create a System of Equations. Create an equation for each element (H, Cl, Na, O) where each term represents the number of atoms of the element in each reactant or product.
Label the chemical Equation - Liveworksheets Label the chemical Equation Label the parts of the chemical Equation ID: 1570824 Language: English School subject: Science Grade/level: 5 Age: 7-9 Main content: Chemical Equation Other contents: NA Add to my workbooks (1) Download file pdf Embed in my website or blog
Science - National Geographic WebExploring the latest in scientific discoveries from prehistoric life to missions to Mars.
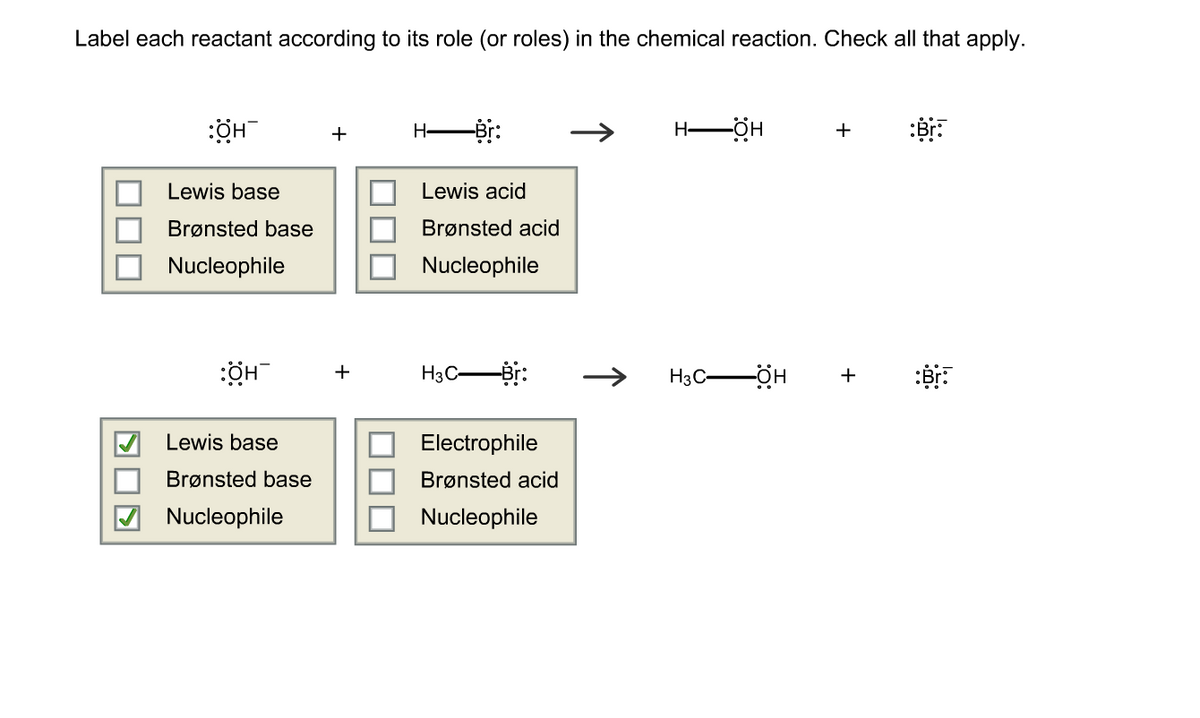
Label Each Reactant and Product in the Given Chemical Reaction reactant. is a substance that is present at the start of a chemical reaction. The substance (s) to the right of the arrow are called. products. . A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product.
Assignment Essays - Best Custom Writing Services WebGet 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
The rate and extent of chemical change - BBC Bitesize WebGCSE Chemistry (Single Science) The rate and extent of chemical change learning resources for adults, children, parents and teachers.
N2 + H2 = NH3 - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a N 2 + b H 2 = c NH 3. Create a System of Equations. Create an equation for each element (N, H) where each term represents the number of atoms of the element in each reactant or product. N: 2 a + 0b = 1 c H: 0a + 2 b = 3 c; Solve ...
How to Write a Chemical Equation (with Pictures) - wikiHow If you want to write a chemical equation, start by writing the chemical formulas of each reactant. Use the prefixes, such as mono-, di-, tri-, and tetra-, to figure out the number of atoms present for each element, and write this number as a subscript for each element. For example, dihydrogen monoxide would be more easily written as H2O.
Al + O2 = Al2O3 - Chemical Equation Balancer WebLabel each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. a Al + b O 2 = c Al 2 O 3. Create a System of Equations. Create an equation for each element (Al, O) where each term represents the number of atoms of the element in each reactant or product. Al: 1 a + 0b = 2 c O: 0a + 2 b = 3 c ...
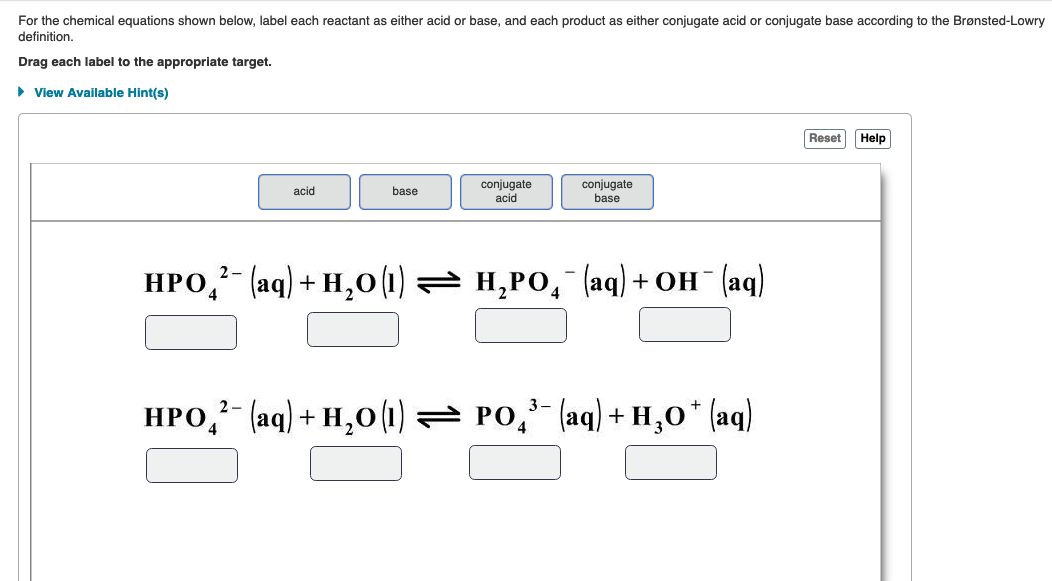










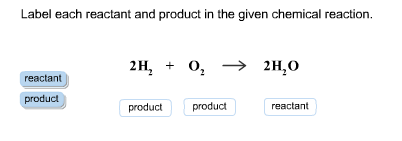
















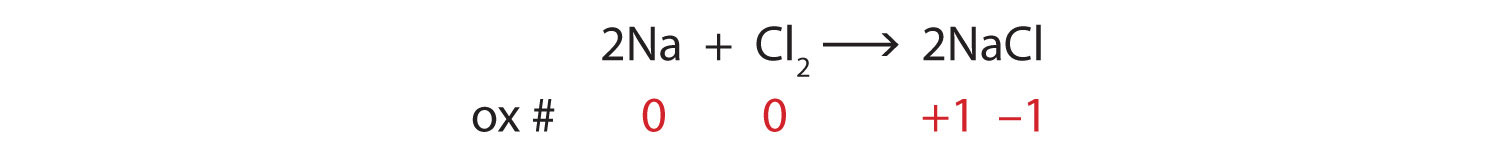
Post a Comment for "43 label chemical equation"